
INTRODUCTION
In recent times, gizzard erosion and ulceration syndrome (GEU) have been very common in poultry at mild to moderate levels. The gizzard, also called a muscular stomach, is composed of a koilin (keratenoid) layer and an underlying mucosa35. Gizzard erosion and ulceration syndrome (GEU) are characterized by erosive lesions in the koilin layer of the gizzard and macroscopic defects in mucosa1. It was described and termed as ‘ventriculitis’2, ‘black vomit’3, ‘vomito negro’4, and ‘Muskelmagenerosionen5, in different published articles. The condition is seen in both layer and broiler chicken, but broilers have a higher incidence.
It usually causes reduced feed intake, growth, poor absorption of nutrients, and persistent diarrhea in birds, which leads to economic losses6. According to research, during GEU development, birds' feed intake and growth were significantly affected, and average body weight gain and feed consumption decreased by up to 12% and 14%, respectively. It affects various domestic birds with a prevalence of 1-50 % depending on species, region, and etiological factors7. In Bangladesh, the prevalence of GE (Gizzard Erosion) in broilers has reached 70.6-87.2%8. GEU has been associated with many diverse factors that have been assumed to play a causative, predisposing, or preventive role.
PREDISPOSING FACTORS OF GIZZARD EROSION
CONGENITAL FACTORS
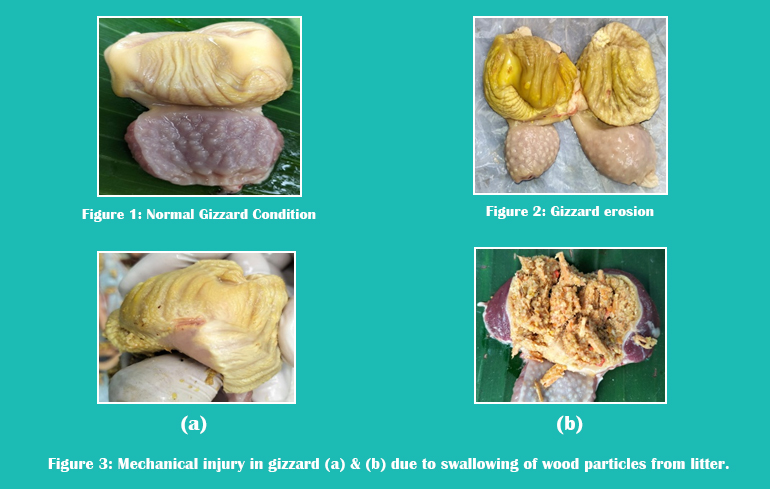
Gizzard and koilin lesions have been found in chick embryos and newly hatched broiler chicks, suggesting that GEU may be congenital and influenced by factors before hatch. GEU in newly hatched chicks may be linked to breeder diet, age, capillary fragility, and transient blood pressure increase during hatching9 (Figs. 1, 2, and 3).
STARVATION
Newly hatched chicks deprived of feed and water experienced an increase in ulcerated gizzards, from 3 to 68% on day 4 post-hatch. Restriction or deprivation of feed and/or water may be associated with increased frequency and severity of lesions whether initiated on hatch day or later in the rearing period9.
NUTRITIONAL DEFICIENCIES
Malnutrition status like deficiency of vitamin B6, vitamin B12, vitamin E, and decreased Sulphur-containing amino acids are associated with gizzard ulceration10. Vitamin B6 plays an important role in synthesizing taurine. Vitamin B6 deficiency results in taurine insufficiency, which results in insufficiency of taurocholic acid, and taurocholic acid means bile acid. Whole bile or bile components (including sodium taurocholate) have a protective effect against gizzard erosion11. Methionine is an intermediate in the biosynthesis of taurine. Inadequate methionine in the diet can cause prevalent and severe gizzard erosion12.
BIOGENIC AMINES AND GIZZEROSINE
Feed microbial contamination will generate most biogenic amines (histamine) which catalyze the secretion of gastric acid13. Several bacteria can convert histidine into histamine, a biogenic amine linked to poor chicken performance. Histamine stimulates proventricular gland receptors, increasing hydrochloric acid secretion and causing superficial gizzard erosion14. Besides this, gizzerosine [2-amino-9-(4-imidazoyl)-7-azanonanoic acid] produced in poor-grade fish meals is an even more effective stimulator of gastric acid secretion in poultry15. Overheating fish meal during
processing the histidine or histamine present in the meal can react with lysine forming a chemical compound called gizzerosine16. Gastric ulcers are initiated and exacerbated by increased acid secretion and a weakened mucosal barrier.
COPPER SULFATE
The adverse effect of high levels of copper sulfate in diets for poultry is gizzard erosion which means ulceration of the lining of the gizzard. It is most likely that the acidic nature of Cu (Copper) or sulfate dissociated from copper sulfate or even both may be responsible. Studies indicated that using extremely high amounts of copper additives (>300 ppm) results in liver cirrhosis, gizzard erosion, and kidney damage, which can seriously affect growth performance17.
RANCID FATS
Rancid fats, particularly in hotter climates where fats readily become rancid if not stored properly; can cause feed passage issues in broilers, leading to lesions like proventriculitis, gizzard erosions, and enteritis18. This process involves the oxidation of fat and fat-soluble compounds, producing free radicals or reactive oxygen molecules.
TANNINS
Tannins, polymeric phenolic compounds found in cereals, legumes, vegetables, and fruits, are a crucial secondary metabolite, consisting of nitrogen-free, hydrolyzed, and condensed forms. Tannins, which contain anti-nutritional content, negatively impact poultry, leading to performance losses such as reduced appetite, reduced feed intake, and poor nutrient absorption. They also cause bone disorders, pathological changes, irritation in the esophagus, necrosis in crops, gizzards, and duodenum, and can cause liver and kidney poisoning19.
ACETYLSALICYLIC ACID & SODIUM SALICYLATES
Acetylsalicylic acid (ASA) and sodium salicylate (SS) are safe for poultry and commonly used in avian medicine due to their anti-inflammatory and analgesic properties. Studies have shown that a dose of 400 mg/kg of either ASA or SS can decrease weight gain and induce gizzard ulceration, which typically occurs in the transition zone between the glandular stomach and gizzard20.
MYCOTOXINS
Trichothecenes are a group of Fusarium mycotoxins, including T-2 Toxin, Monoacetoxyscirpenol (MAS), Diacetoxyscirpenol (DAS), Deoxynivalenol (DON), and HT-2 toxins, which can cause gizzard erosion, oral lesions, and intestinal hemorrhage in the gastrointestinal tract. T-2 toxin and DAS are the most caustic, causing gizzard erosion. Cyclopiazonic acid (CPA) also causes mucosal necrosis in the gizzard21. Feed-borne mycotoxins like FB1, DON, AFB1, and OTA negatively impact intestinal epithelium integrity and cause necrotic enteritis and GEU in broiler chicks. Studies have reported impaired feed intake and growth in chickens during GEU development6.
VIRAL CAUSES
Fowl adenovirus (FAV) is found in chickens, as evidenced by antibody surveys and high isolation rates in both healthy and sick birds. FAV is linked to a variety of diseases such as IBH, HPS, respiratory disease, necrotizing pancreatitis, and gizzard erosion22. Adenoviral inclusions in chicken gizzard epithelium show GEU lesions. Both vertical and horizontal transmission are crucial for adenovirus spread. Adenoviral gizzard erosion can be reproduced by horizontal transmission, and broilers affected usually show no clinical signs, mainly detected at slaughter. Gross lesions in the gizzard comprised mucosal injury that was ulcerative or necrotizing and koilin layer separation. According to histopathology, there was necrotizing ventriculitis with basophilic intranuclear inclusion bodies in the epithelial cells. Utilizing immunohistochemistry, egg culture, and electron microscopy, adenovirus-like particles were found22. Gizzard lesions often contain virus strains from serotype 1 of fowl adenovirus A, with FAdV-4 and FAdV-8 being the aetiologic agents in a few cases22.
BACTERIAL CAUSES
Bacillus cereus (B. cereus) isolates have been found to causegizzard erosion and ulceration syndrome (GEU) in birds, potentially causing vomiting, diarrhea, or ulceration23. B. cereus can tolerate low pH by forming spores attached to the hydrophobic layer of gastric epithelium25. The koilin layer of the gizzard contains leucine proteins and arginine, which may help form biofilms, leading to stomach acid tolerance and metabolism23. Enterotoxins produced by B. cereus, Hbl, and Cytk genes, can cause dermonecrotic, cytotoxic, and hemolytic effects24. These toxins can reach their highest level during the late growth phase, causing damage to the koilin layer and mucosa of the gizzard, leading to long-term ulceration and diarrhea23.
Clostridium perfringens was discovered to be an opportunistic bacterium in commercial chickens with gizzard ulcerations, and the severity of the lesions was observed to considerably increase caecal numbers26.
DIFFERENTIAL DIAGNOSIS
Establishing a differential diagnosis between agents causing gizzard erosion and ulcerative syndrome in poultry is challenging. Gizzard erosion can be caused by multiple factors simultaneously. To address this, focus on non-infectious factors such as breeder management, Adenoviral vaccination, feed formulation, anti-nutritional factor(tannin), hatchery management, litter management, starvation history, and commercial poultry feed formulation (especially, vitamin-mineral premix, amino acids, quality and quantity of fish meal and amount of copper sulphate). Deviation from any of these factors can lead to gizzard erosion.
Adenoviruses can cause GE, causing macroscopic damage in other organs. The liver is pale, friable, and enlarged, with focal or diffuse necrosis. Degenerative and necrotic changes in the liver cause dysfunction and decrease blood osmotic pressure, leading to straw-colored fluid accumulation in the pericardial sac27. Basophilic intranuclear inclusion bodies are present in hepatocytes, surrounded by a clear halo or filling the entire nucleus which are absent from other casual agents28.
Mycotoxins causing gizzard erosion (GE) typically cause macroscopic damage in other organs, including bursal atrophy, enteritis, diarrhea, and mouth lesions. Under field conditions, mycotoxins affect multiple organs, and typical changes in organs like the thymus should be present when T2 toxin or DAS cause gizzard damage. Mycotoxin levels in feed can be identified by High-Performance Liquid Chromatograph (HPLC) which are rapid analysis, and detection at low levels with improved accuracy, good repeatability, and reproducibility29.
CONTROL STRATEGY
- The inclusion of grit and at least 20% larger cereal particles larger than 1 mm in size in poultry diets can positively impact the development and functioning of gizzards and reduce the frequency and severity of GEU lesions30.
- The study found that non-soluble fibers significantly impact the structure and function of gizzards, with at least 3% coarse fibers in feed increasing gizzard weight and reducing pH, suggesting fibers may prevent GEU damage30. To improve gizzard condition to eliminate rancid fat and tannins like antinutritional factors from diet.
- Proper monitoring is required to avoid starvation in the early stages of chicks. Good brooding management, a proper number of feeders and drinkers, and a balanced diet can improve the feed intake of chicks. Another thing to be aware of is that farmers should avoid using sawdust as litter material because chicks can mistake sawdust for feed, and in the process, they consume it in good quantity, which leads to gastrointestinal impaction, mechanical injury in the gizzard and ultimately results in death.
- Broiler feed formulation should prioritize vitamin E, B6, B12, and sulfur-containing amino acids to prevent nutritional deficiencies. Vitamin E is protective against GEU if there are high polyunsaturated fatty acids in feed10. Vitamin B6 and methionine are crucial in broiler nutrition as they aid in taurocholic acid or bile acid synthesis, preventing gizzard erosion31.
- To reduce the effects of biogenic amine or gizzerosine, low-quality fish meal should be avoided. In the modern processing of fishmeal, the drying process has changed large extent, using low temperatures to reduce gizzerosine levels.
- Judicious use of salicylates as drinking water medications and avoid overdoses. Copper sulfate is used very carefully as a water sanitizer and antifungal in feed and feed ingredients. An important factor to take into consideration to prevent this problem is to avoid overdosing and to choose a reliable source that will not form clumps in the feed.
- Feed-borne mycotoxins can cause necrotic enteritis and GEU in broiler chicks, affecting intestinal epithelium integrity7. Using broad-spectrum mycotoxin binder can reduce mycotoxin-related risks in animal nutrition and production, as minimal/negligible binding of Vitamin B6 and Vitamin E and non-digestible mycotoxin binders facilitate excretion through excreta without gastrointestinal tract absorption.
- The spread of avian adenovirus among flocks can be reduced through awareness and proper biosecurity measures. Separating infected and non-infected breeding flocks and vaccination of affected birds can be done. Inclusion body hepatitis vaccination to parent stock has been shown to pass protective titers to progeny and should be considered. Commercial vaccines are commonly used for serotypes 4 and 8, and broilers are vaccinated at <10 days of age when their parents lack serotype-specific antibodies32.
- The antimicrobial factor produced by Bacillus subtilis PB6 is broadly active against various strains of Clostridium species33.
CONCLUSION
Gizzard erosion and ulceration syndrome (GEU) is a global issue affecting commercial poultry flocks, affecting digestion and immune responses. This poses a threat to food safety and public health. GEU is caused by a combination of viral, nutritional, and toxic agents, making it difficult to identify a single etiologic agent. To prevent and control GEU, long-term monitoring and surveillance are necessary, along with effective differential diagnosis by experienced veterinarians.
REFERENCES
- Anne-Gerd Gjevre, MagneKaldhusdal& Gunnar Sundstøl Eriksen (2013) Gizzard erosion and ulceration syndrome in chickens and turkeys: a review of causal or predisposing factors, Avian Pathology, 42:4, 297-303, DOI: 10.1080/03079457.2013.
- Randall, C.J. & Reece, R.L. (1996). Colour Atlas of Avian Histopathology 1st edn. London: Mosby-Wolfe.
- Herrera, C., Tello, M., Ibanez, I., Valenzuela, O. & Olivares, L. (1999). Synthesis of gizzerosine, a toxic substance found in fish meal. Boletin de la Sociedad Chilena de Quimica, 44, 117-121.
- Fulton, R.M. (2008). Other toxins and poisons. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan, & D.E. Swayne (Eds.). Diseases of Poultry 12th edn (pp. 1236). Ames: Blackwel.
- Fries, R., Bergmann, V. &Fehlhaber, K. (2001). Praxis der Geflu¨geluntersuchung. Hannover, Germany: Schlu¨tersche GmbH & Co KG, Verlag und Druckere.
- Wang Y et al., 2021. Contamination with fumonisin B and deoxynivalenol is a threat to egg safety and contributes to gizzard ulcerations of newborn chickens. Frontiers in Microbiology 12: 676671.
- Haque MA, Zuo Z and He C, 2023. Pathogenesis of Gizzard Erosion and Ulcerations and Comprehensive Control Strategy in Poultry. In: Abbas RZ, Saeed NM, Younus M, Aguilar-Marcelino L and Khan A (eds), One Health Triad, Unique Scientific Publishers, Faisalabad, Pakistan, Vol. 2, pp: 129-137
- Moula MM et al., 2020. Evaluation of broiler health status through flock health monitoring program in Bangladesh. Poultry Science Journal 8: 63-72.
- Good, R.E., Hetrick, J.M. & Hanley, J.E. (1968). Observations on gizzard ulcers in baby chicks. Avian Diseases, 12, 327-331.
- Scott, M.L. (1985). Gizzard erosion. After fifty years of mystery, some answers. Animal Health and Nutrition, 2229.
- Almquist, H.J. (1938b). Influence of bile acids on erosions of the chick gizzard lining. Science, 87, 538.
- Miller, D., Bauersfeld, P.E., Jr., Biddle, G.N. & Fortner, A. (1975). Effect of sulfur-containing dietary supplements on gizzard lining erosions. Poultry Science, 54, 428435
- Hungerford, J.M. (2010). Scombroid poisoning: a review. Toxicon, 15, 231-243.
- Harry, E.G. & Tucker, J.F. (1976). The effect of orally administered histamine on the weight gain and development of gizzard lesions in chicks. The Veterinary Record, 99, 206207.
- Smith TK et al., 2000. Feed-borne biogenic amines: natural toxicants or growth promotors? Proceedings of “the 5th International Symposium on Aquaculture Nutrition”. Merida, Yucatan, Mexico; 19-22 Nov 2000, pp: 24-32.
- Masumura, T., Horaguchi, H., Horikawa, H. &Sugahara, M. (1981). Gizzard erosion and ulceration in broilers. 3. Toxic substances in fish meal. Japanese Poultry Science, 18, 98-104.
- Luo XG, Ji F, Lin YX, Steward FA, Lu L, Liu B, et al. Effects of dietary supplementation with copper sulfate or tribasic copper chloride on broiler performance, relative copper bioavailability, and oxidation stability of vitamin E in feed. Poult Sci. 2005, 84(6): 888–893. https://doi.org/10.1093/ps/84.6.888 PMID: 15971525
- Butcher, G.D., Nilipour, A.H. and Miles, R.D., 2002. Feed passage in broilers-A complex problem. University of Florida. Panama. Republic of Panama.
- Çalıșlar, S., 2018. Effects of tannins on poultry nutrition. KSÜ TarimveDogaDergisi, 21(4), pp.615-623.
- Poniak , M. witaa , K. Bobrek , S. Graczyk& S. Dzimira (2012) Adverse effects associated with high-dose acetylsalicylic acid and sodium salicylate treatment in broilers, British Poultry Science, 53:6, 777-783, DOI: 10.1080/00071668.2012.745929
- Kamalavenkatesh, P., Vairamuthu, S., Balachandran, C., Manohar, B.M. & Raj, G.D. (2005). Immunopathological effect of the mycotoxins cyclopiazonic acid and T-2 toxin on broiler chicken. Mycopathologia, 159, 273279
- Ono, M., Okuda, Y., Yazawa, S., Imai, Y., Shibata, I., Sato, S. and Okada, K., 2003. Adenoviral gizzard erosion in commercial broiler chickens. Veterinary pathology, 40(3), pp.294-303.
- Haque MA et al., 2021a. Pathogenicity of feed-borne Bacillus cereus and its implication on food safety. Agrobiological Records 3: 1-16.
- Ehling-Schulz M et al., 2006. Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiology Letters 260: 232–240.
- Tsilia V et al., 2016. Bacillus cereus NVH 0500/00 can adhere to mucin but cannot produce enterotoxins during gastrointestinal simulation. Applied and Environmental Microbiology 82: 289 –296.
- Dinev, I. (2010). Enzootic outbreak of necrotic gastritis associated with Clostridium perfringens in broiler chickens. Avian Pathology, 39, 7-10.
- Sharma S, Asrani RK, Singh G, Gulathi BR, Patil PK, Gupta VK et al. Outbreak of hydropericardium syndrome associated with ascites and liver rupture in caged broilers. Vet Res Int. 2014; 2:33-45.
- Abdul Aziz TA, Hasan SY. Hydropericardium syndrome in broiler chickens: its contagious nature and pathology. Res Vet Sci. 1995; 59:219-221.
- Pittet, A. (2005) Modern methods and trends in mycotoxin analysis. MitteilungenausLebensmitteluntersuchung und Hygiene 96, 424–444.
- Svihus, B. (2011). The gizzard: function, influence of diet structure and effects on nutrient availability. World’s Poultry Science Journal, 67, 207-223.
- Miller, D., Bauersfeld, P.E., Jr., Biddle, G.N. & Fortner, A. (1975). Effect of sulfur-containing dietary supplements on gizzard lining erosions. Poultry Science, 54, 428-435.
- Cowen, B. (1992). Inclusion body hepatitis-anemia and hydropericardium syndromes: aetiology and control. Worlds Poult. Sci. J. 48:247-254.
- Teo A.Y. and Tan H.M. 2005. Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated
- Contreras M, 2016. Gizzard erosion in broilers and the impact of avian adenoviruses. International Meat Topics 7: 27-28.
- Kemin Internal Documents- IMG-KAI-00692/00693/00694/00695.






